The First True Innovation in Cannulation Since 1954
The world of medicine prides itself on innovation—but when it comes to intravenous (IV)
access, we are stuck in the past. The "Safety IV Cannula" has long been marketed as a modern solution for patient safety. But beneath the glossy marketing lies a stagnant technique that has not addressed the biggest pain point in cannulation: successful insertion on the first attempt.
It’s time for manufacturers, regulators, and healthcare providers to look beyond hollow promises. It’s time for Venaican—a spring-loaded, self-introducing IV cannula that prioritizes patient comfort, clinical efficiency, and infection control.
What is a Safety IV Cannula—and What Isn’t It?
The Safety IV Cannula was introduced primarily to reduce needle-stick injuries, a legitimate concern for healthcare workers. The FDA backed its use in the USA, and it has since become standard in many countries. At its core, it’s a regular IV cannula with a safety clip (SIP–Sharps Injury Protection) that retracts or shields the needle after insertion.
This safety mechanism is helpful, but let’s be clear: it only protects after the damage is already done. The insertion technique—the act of getting the cannula into the vein remains unchanged since 1954.
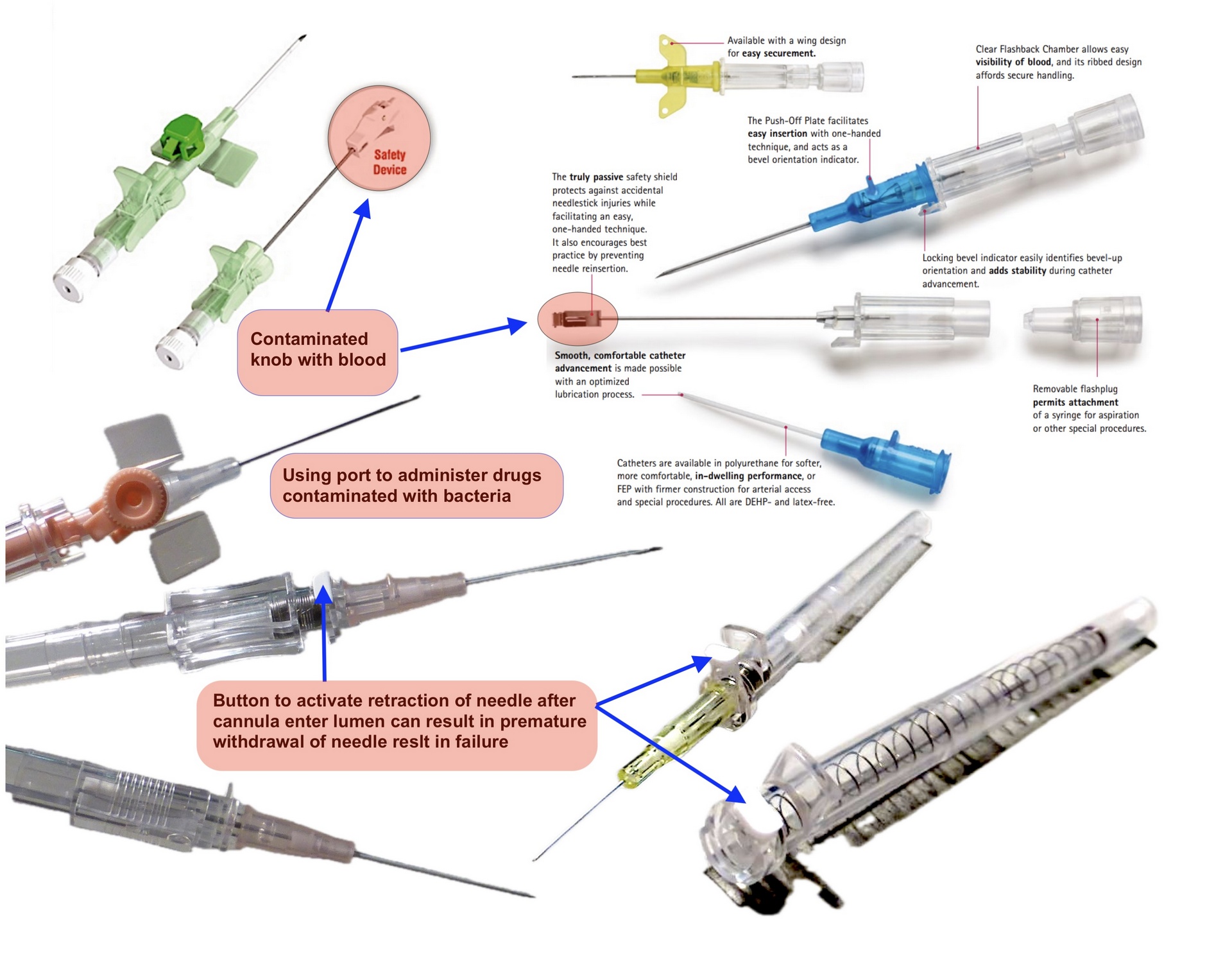
Here’s what that means in practice:
- Multiple failed attempts to access a vein.
- Trauma to patients, especially children, the elderly, or the dehydrated.
- Increased risk of infection with every failed attempt.
- Emotional and physical exhaustion for healthcare staff.
Yet none of these are addressed by the so-called "safety" cannula.
Why the World Needs VenaiCan—Now
During the Ebola epidemic and most recently during the COVID-19 pandemic, frontline doctors wearing PPE struggled to cannulate dehydrated, collapsed veins. Repeated pricks and failures led to poor outcomes and suffering—not because of bad doctors, but because the tools had failed them. According to WHO, the next pandemic is said to be 10 times worse, and we know this is not a threat to humanity, but members of medical profession. That’s why I developed VenaiCan, the first spring-loaded, self-introducing cannula designed to:
- Ensure successful vein entry on the first attempt.
- Eliminate the need for high skill levels, allowing even non-doctors to use it safely in emergencies.
- Reduce trauma, infection risks, and delays in critical care.
- Restore dignity and autonomy to patients—especially in pandemics, war zones, and rural or under-resourced settings.
Unlike the Safety Cannula, which only protect healthcare workers acts after insertion, and has no true benefit, but expensive for patients, and healthcare providers. VenaiCan transforms the insertion process, reduce cost, and environmentally friendly.
The Hidden Agenda Behind the "Safety" Cannula
Let’s not ignore the commercial history: The safety cannula was heavily patented and monopolized by manufacturers to maintain market dominance, not necessarily to advance patient care. By focusing only on post-use safety, they avoided real innovation while capturing global markets through regulatory influence.
But now, that monopoly is hurting us. We're overdue for a product that doesn’t just protect after cannulation, but ensures the cannula is successfully introduced without trauma in the first place.
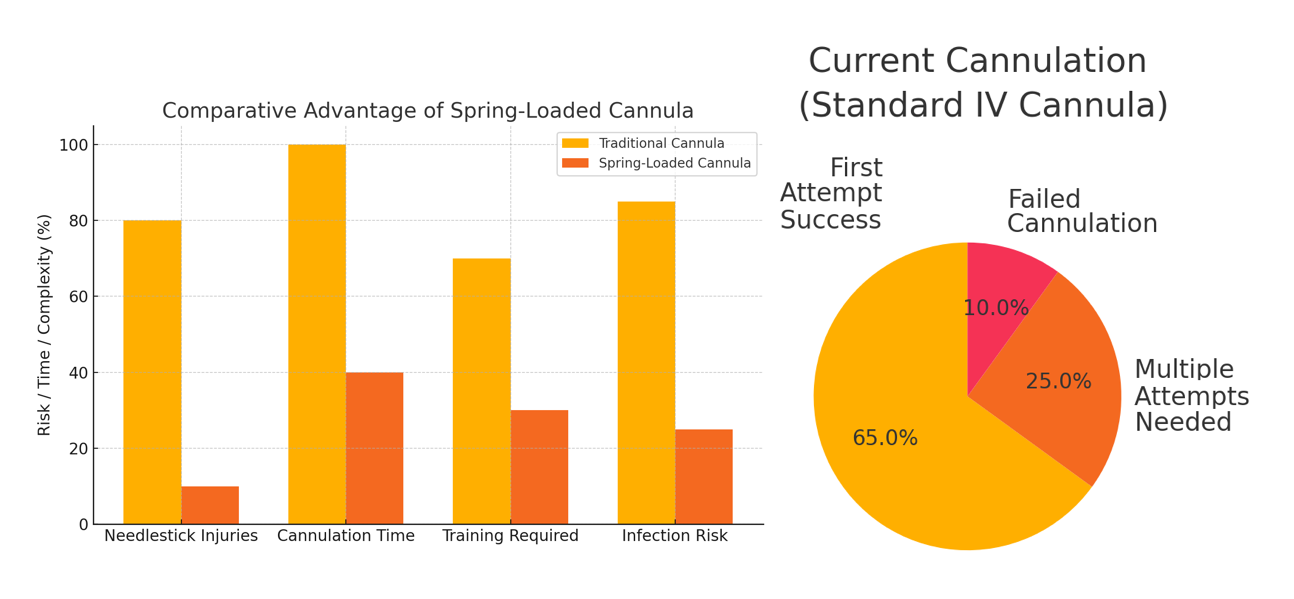
The Future of Cannulation: Patient-Centered, Not Patent-Driven
VenaiCan is not just a medical device—it’s a revolution in how we treat patients, especially the most vulnerable. It empowers:
- Patients to self-cannulate during emergencies.
- Healthcare workers to operate under pressure without stress.
- Systems to reduce iatrogenic infections and mis-cannulations.
Let’s not settle for half-solutions anymore. The world needs real innovation, not marketing jargon.
Self-Introducing Spring-Loaded Cannula with Manual Needle Retraction and Integrated Blood Collection Chamber
The present invention relates to a novel intravenous (IV) and arterial access device comprising a spring-loaded, self-introducing cannula with a manually retractable introducer needle and an integrated blood collection chamber. The device addresses critical limitations of current safety cannulae by enabling precise, single-attempt cannulation, particularly in high-risk, low-resource, or high-stress environments such as during pandemics, military operations, or intensive care settings.
Upon release of a user-controlled plunger restrainer, a pre-tensioned spring gently advances the cannula over a fixed needle tip into the vessel, ensuring stable and accurate insertion with minimal trauma. Following successful cannulation, the needle and attached blood collection chamber can be manually retracted to ensure controlled withdrawal and reduce sharps-related injury. This invention improves first-attempt success rates, reduces procedural time, and minimizes complications such as double puncture, infiltration, or nosocomial infections. It also eliminates the need for advanced skill, enabling use by non-specialists or even trained patients in emergencies.
Field of the Invention:
This invention relates to medical devices, specifically to intravenous and arterial cannulation systems used for vascular access, drug administration, and blood collection. The invention addresses longstanding challenges in safe and effective cannula insertion and needle retraction mechanisms.
Background of the Invention:
Traditional cannulation methods involve manually advancing a plastic cannula over a sharp needle into a vein or artery. This technique, first developed in the mid-20th century, often requires skilled handling and frequently results in failed or multiple attempts, causing trauma and increased infection risk. The introduction of safety cannulae focused on needle-stick injury prevention post-cannulation but failed to address the difficulty of inserting the cannula in a single attempt. Furthermore, improper aseptic practices, particularly failure to observe biocide drying times, have contributed to an increase in iatrogenic infections and sepsis.
Summary of the Invention:
The invention provides a spring-assisted cannula introduction system designed to enter the vein or artery in a single, controlled motion. A locking plunger mechanism secures the cannula in position until released by the user. Upon release, the spring mechanism advances the cannula forward over the needle tip, allowing for accurate vein entry.
After insertion, a manual retraction system enables safe withdrawal of the introducer needle and blood chamber into a protective housing, reducing exposure to sharps injuries. The invention is compatible with standard cannula dimensions and IV systems and can be operated in both manual and assisted modes, ensuring universal applicability in diverse healthcare environments.
Call to Action
Think, and don't regreat when your loved ones or you in hospital with infections that cannot be cured. The doctors may promise miricle cures, knowing there are non. Only thing they can offer is to alliviate pain and suffering if they can have venous access to administer drugs, and fluids.
During Ebola epedemic spreading in West Africa, doctors contacted me asking about the "Spring-loded Cathter Introducer I patented". Unfortunatly, it was not manufactured, because the cannula manufacturers had invested in the so called "Saftey Cannula" and were not keen to "De-Skill" the end users.
I urge manufacturers, hospital procurement heads, and medical innovators to stop glorifying outdated safety devices and start supporting first-attempt success, real infection prevention, and trauma-free cannulation.
Let’s evolve. Let’s build. Let’s make VenaiCan the new global standard.
Join us in putting patients first—where they belong.